Cryogenics
Cryogenics is defined as the branch of physics dealing with the production of very low temperatures and their effects on the matter but more generally, cryogenics is the science and its application to physical phenomena ocurring below 120 K (-153 °C). This temperature of 120 K represents the limit where main atmospheric gases begins to liquefy (methan, oxygen, argon, nitrogen, argon, neon, hydrogen and helium).
Helium
Most of cryogenic systems use a cryogenic fluid where the latent heat of vaporization is used to maintain an object at
a constant temperature. Consequently, the choice of the cryogenic fluid depends directly of the desired temperature.
If the desired temperature is below 5 K (-263 °C), the only possible solution is the Helium because helium starts to liquefy
around 5 K and all other elements are solid at this temperature. Note that helium is the only element where there is no solid phase
at low temperatures when pressure is kept below 25 bar.
Moreover, helium demonstrates another state called superfluid when it is below 2.17 K.
This state is designated by "He II", by opposition to the normal fluid "He I".
This state is a quantum state of the matter where all the molecules behave in the same manner.
It results extraordinary properties as the absence of viscosity or the perfect ability to transport the heat.
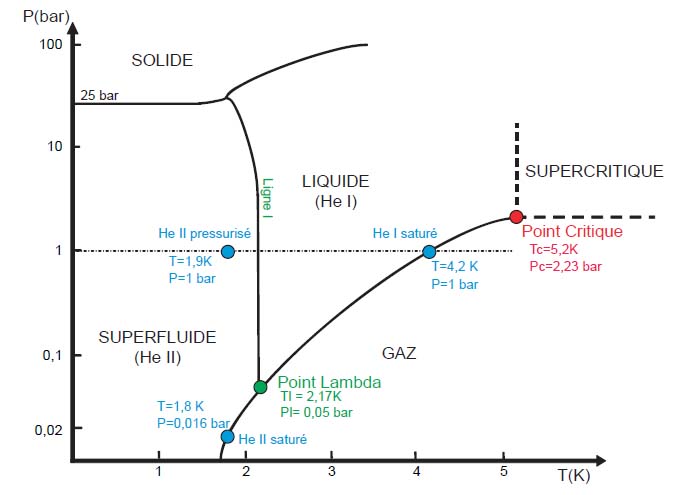
Phase diagram of helium-4
Cryogenics for the LHC accelerator
Cryogenics represents one of the biggest challenges of the
particle accelerator LHC (Large Hadron Collider). The LHC is composed of 1700
Nb-Ti superconducting magnets maintained at 1.9 K (-271.25 °C) along a ring of 27km.
To achieve this challenge, LHC makes use of superfluid helium due to its unique thermal
transport and viscosity properties.
Some LHC cryogenics key figures:
- Cold mass at 1.9 K = 36 000 tons (3x Eiffel Towers)
- Helium inventory = 150 tons (100 millions of balloons)
- Electrical power consumption = 36 MW (3x TGV Eurostar)
- Control system = 60 000 sensors and actuators
- Overall availaibility = 97 % over ~230 days of operation per year
References
- P. Lebrun. Introduction to cryogenics. CAS - CERN Accelerator School : Vacuum in Accelerators, pp.15-30, 2006.
- B. Bradu. La premiere liquefaction de l’helium par Heike Kamerlingh Onnes. BibNum, CERIMES, 2008.
- S. Balibar. Qui a découvert la superfluidité ?. Laboratoire de Physique Statistique de l’Ecole Normale Supérieure, 2001.
- P. Lebrun. Cryogenics for the Large Hadron Collider. LHC-Project-Report-338, 1999.
© Copyright Benjamin Bradu's HomePage all rights reserved
Last update: December 2021
 HOME
CRYOGENICS
HOME
CRYOGENICS